
Juliette Reeves Nutritionist and Dental Hygienist
BDJ Team volume 6, Article number: 19040 (2019) | Download Citation Dental hygienist Juliette Reeves was among the delegates at the recent British Society of Periodontology (BSP) conference and provides BDJ Team readers with a summary of the latest thinking on nutrition and systemic diseases as risk factors in the aetiology of periodontal disease.
Nutrition
A balanced diet is made up macronutrients (fats, carbohydrates and proteins) and micronutrients (vitamins, minerals and trace elements) all of which are involved in human growth and development, including metabolic regulation and other biological processes. Deficiency therefore results in a wide variety of systemic and chronic inflammatory diseases. The role of oxidative stress was highlighted by Dr Milward as playing a central role in the pathogenesis of chronic inflammatory diseases2 and has also been proposed as a common factor in the association of periodontitis and systemic diseases.3,4 Oxidative stress is thought to make a significant contribution to all inflammatory diseases, including inflammatory periodontal disease. An increasing body of evidence is emerging to implicate free radical activity in the pathogenesis of periodontal breakdown.5 In susceptible individuals, periodontal disease is characterised by a dysregulated host response to pathogenic bacteria, resulting in a heightened inflammatory response. It is thought that 20% of the tissue response is mediated by bacterial burden and 80% of tissue destruction is mediated by the host response.4 Oxidative stress can be defined as an imbalance between damaging reactive oxygen species (ROS) and protective antioxidant (AOX) compounds. (Fig 1) Antioxidants are substances that protect other cells in the body from damaging oxidation reactions which occur primarily as part of the inflammatory response.
Dr Mike Milward quoted the WHO definition of nutrition as ‘an adequate, well balanced diet combined with regular physical activity. Poor nutrition can lead to reduced immunity, increased susceptibility to disease, impaired physical and mental development, and reduced productivity.’1
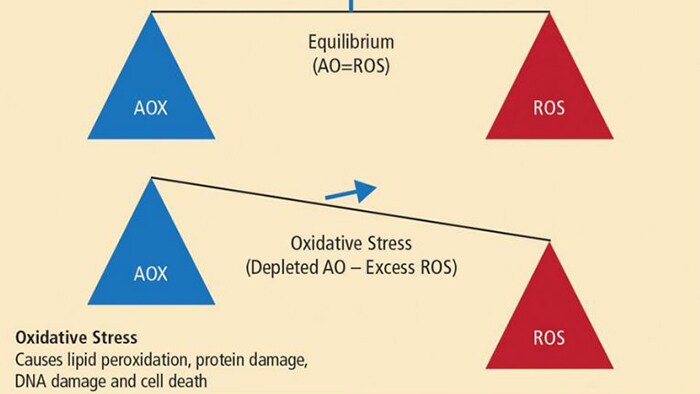
Oxidative burst is part of the inflammatory response and the physiological function of polymorphonuclear leukocytes (PMLs), which, during phagocytosis, results in massive production and release of reactive oxygen species (ROS). This response is needed to destroy invading micro- organisms, but, over a prolonged period, exerts oxidative stress on otherwise healthy tissues. Research has suggested that low levels of antioxidants may be implicated in the susceptibility and progression of chronic periodontal disease and that high concentrations of antioxidants in health may represent an important anti-inflammatory defence system in the progression of inflammatory periodontal disease.6 Earlier research has also suggested that lowered local and systemic antioxidant capacity is a feature of periodontal disease.7 The optimal function of the host defence system depends, therefore, on an adequate supply of antioxidant micronutrients.8 The antioxidant micronutrients are important not only for limiting oxidative damage and tissue damage, but also in preventing increased cytokine production, which is a result of prolonged activation of the immune response. Adequate host defence activity critically depends upon the micronutrient status of an individual, particularly the oxidant- antioxidant balance.8 Food sources that contain antioxidants including vitamin A, C, E, selenium and zinc are found in fruits and vegetables, nuts, seeds, oily fish and wholegrains.
Diabetes and obesity
Antioxidant rich foods Vegetables: sweet potato, carrots, red and yellow peppers, leeks, courgettes, tomatoes Fruits: apricots, mango, cantaloupe melon, pink grapefruit, avocados Nuts: brazil nuts, almonds, cashews, mixed nuts and raisins, hazelnuts Seeds: sunflower, sesame, pumpkin, tahini paste Oily fish: salmon, mackerel, herring, tuna, sardines, trout Meat: Beef, chicken, pork Nutrition risk factor check list: Medical history: check for risk of diabetes, cardio-vascular disease, digestion and absorption issues such as Crohn’s, diverticulitis and IBS Dietary habits: reduce/avoid: refined foods, sugars, saturated fats. Increase: fruit, vegetables, lean protein, nuts, seeds, oily fish and wholegrains Lifestyle: smoking, alcohol consumption, sedentary lifestyle, exposure to sunlight, isolation/ loneliness in older people The World Health Organisation (WHO) reports that rates of obesity worldwide have tripled since 1975 with 39% of adults aged 18 and over being classified as overweight and 13% of adults being obese.9 28% of the UK adult population has been classified as obese.10 The cause was identified as increased energy intake from foods dense in fat, sugar and salt. This coupled with a decrease in physical activity and the sedentary nature of many forms of work, has led to the statistics seen today. The relationship with periodontal disease is associated with shared factors in the immuno- inflammatory cascade. The association is mediated by adipose tissue which acts like an endocrine organ producing leptin and contributing to elevated levels of pro-inflammatory cytokines, such as tumour necrosis factor (TNF) and interleukin-6 (IL-6).11 Being overweight has been positively associated with periodontitis in adults12 with a 35% increased risk in obese patients13 and a three fold increased risk in type 2 diabetes patients.14 Diabetes impacts on the ‘whole person’ and research shows that patients with diabetes and severe periodontal disease are three times more likely to develop end stage renal failure15 and have a higher risk of death from ischaemic heart disease.16 The treatment of periodontal disease is associated with a reduction in HbA1c.17 Each 1% reduction in HbA1c is associated with a 21% reduction in deaths related to diabetes and a 37% reduction for myocardial complications.18 This reinforces the European Federation of Periodontology (EFP) manifesto call to all dental professionals to act in the prevention, early diagnosis and effective treatment of periodontal disease in order to combat the devastating oral and general health effects for the individual and society.19 The Joint EFP-IDF workshop on perio diabetes20 highlighted the role the dental team can play in treating the diabetes patient and the advantages of interdisciplinary care amongst the dental and medical professions.21
There are a number of systemic diseases associated with an increased risk for periodontal disease. Professor Philip Preshaw presented the latest statistics and evidence of the relationship between obesity, diabetes and periodontal disease.
Vitamin D and periodontal disease
Vitamin D deficiency, based on bone health research outcomes, is set at a serum 25(OH)D threshold of <25nmol/L - 30nmol/L22 as a population based recommendation. It is recognised however, that at risk groups such as those with dark skin and those with health issues, are likely to require a threshold of 50nmol/L. A review of standardised data show that across Europe, 13% of the population fall below the 25nmol/L threshold with 16% of adults in the UK showing serum levels of 25(OH)D below 25nmol/L and 20% below 30nmol/L respectively.23,24 Worldwide, the data shows 120 million individuals fall below the 30nmol/L threshold.25 Vitamin D food sources Oily fish: salmon, herring, tuna, sardines, mackerel Egg yolks, butter, beef Cheese, mushrooms/fungi Fortified foods, margarines, cereals, milk, yoghurts The major source of Vitamin D is the conversion of 7-dehydrocholesterol to Vitamin D3 via exposure of skin to UVB radiation (sunlight). In some parts of the globe availability to UVB is limited to 2-8 months of the year resulting in some populations being unable to synthesise Vitamin D for over half of the year.26 The 2016 SACN report on Vitamin D and Health recommends a daily intake of 10ug/d.22 Data shows that the mean UK intake of Vitamin D is 2-3 ug/d,22 indicating that the majority of the UK population is receiving intakes below the Estimated Average Requirement (EAR). The importance of dietary intake and food sources cannot be underestimated, however. Food sources of Vitamin D are limited and whist population statistics show improved Vitamin D status with supplementation,27,28 data collected from the National Health and Nutrition Examination Survey (NHANES) in the US show that only 30% of the population take vitamin supplementation, leaving two thirds of the population with an intake below the recommended EAR.29 Based on randomised controlled trials in population studies, the way forward, therefore, may be to increase the diversity of food products fortified with Vitamin D.30,31,32,33 Professor Thomas Dietrich considered the effects of Vitamin D deficiency on the periodontium, looking at health outcomes beyond bone tissue, including inflammatory diseases such as periodontal disease. In the first study carried out in 2004 using NHANES data, Dietrich et al34 found no difference in attachment loss (AL) between Vitamin D sufficient and Vitamin D deficient groups in men and women aged 20-50 years. However in men and women aged over 50 years, AL was significantly associated with serum 25(OH)D3 concentrations independently of bone mineral density (BMD). The question of whether these clinical findings could be attributed to immune- modulatory effects was considered. Further studies showed an association between serum concentrations of 25(OH)D and gingival inflammation,35 and when considering tooth loss, subjects were 20% less likely to lose teeth with sufficient 25(OH)D serum concentrations.36 Zahn was also able to show subjects 14% less likely to lose teeth over a 5 year period with serum 25(OH)D concentrations greater than 30nmol/L.37 However, a recently published38 systematic review of Vitamin D intake and the risk of periodontal disease was unable to support or refute the association pending more longitudinal clinical studies and standardised periodontal and Vitamin D data.
Vitamin D has long been associated with bone health and along with calcium benefits all population groups. As one of the authors of the 2016 Scientific Advisory Committee on Nutrition (SACN) Vitamin D and Health report, Professor Kevin Cashman considered the role of dietary sources of Vitamin D and recommended intake levels.
Lifestyle patterns
Professor Jayne Woodside presented data concerning diet and lifestyle patterns and chronic disease risk. The Global Burden of Disease39 study cites diet and lifestyle factors as being leading risk factors causing early death and disability. These include smoking as the number one factor, alcohol intake, high blood pressure and high blood sugar levels – all of which are attributable to diet and lifestyle. Over the last two years the Eat Well Guide40 has been revised and updated, reducing the recommended intake of free sugars to no more than 5% of dietary energy and increasing fibre intake to 30g a day for adults. There has been a shift in research patterns over the last few years looking at dietary patterns instead of studying individual nutrients as it is recognised that food and nutrient intakes are synergistic in the effect on health and disease.
Key points
It is now recognised that physical activity also plays a part in health and disease and this is being incorporated into food pyramid graphics.41 The Mediterranean Diet (TMD) has recently been updated to reflect cultural and socio-economic variations.42 There is an increasing body of high quality evidence to support the effects of TMD in decreasing cardiovascular disease and diabetes in high risk groups.43,44 Sofi et al45report that for each two point increase in TMD score, a 5% decrease in overall cancer statistics was observed. The discussion surrounding the question of how to encourage dietary changes at a population level included raising awareness of the health benefits, educating the public, increasing the availability of healthy options outside of the home and working with government to encourage change. More studies are needed to encourage behaviour change in ‘at risk’ populations. When addressing the needs of our elderly population, Professor Marion Hetherington discussed the importance of orosensory exposure and its influence on satiety. The 2013 Malnutrition Taskforce46 reports 1 in 10 people aged 65 and over living in the community are malnourished or at risk of malnutrition and one in three older adults in care homes are at risk of malnutrition.47 Launa et al48 demonstrated that increasing age is associated with reduced capabilities and that grip showed a strong correlation to orofacial muscle strength. The perceived difficulties with eating certain foods resulted in these foods being avoided in older adults with the lowest eating capability scores. Appetite decreases with increasing age along with a diminished sense of taste and smell. Other factors including dry mouth, poverty, loneliness and depression all impact on the nutritional habits of older people. Presenting foods in an easy to access format such as soft foods, serving foods with sauces and in smaller more frequent portions and enriching foods with high protein products to aid in tissue growth and repair are some of the methods recommended to encourage nutrient intake in older adults. The ultimate goal being to enhance the health, wellbeing and quality of life of older adults. As a profession we are in a prime position to encourage healthy lifestyle practices in our patients, not only in an effort to improve their periodontal health but also to enhance their overall health, wellbeing and quality of life.
This article has been supported by the BSP and Philips Oral Healthcare and its Shine On campaign which highlights exemplary work by Dental Hygienists. Juliette Reeves is a dental hygienist and trained nutritionist with over thirty years experience. She has written and lectured internationally over the last eighteen years on the systemic associations between nutrition and oral health with a particular focus on chronic inflammation. She is Clinical Director of Perio-Nutrition and an elected executive committee member to the British Society of Dental Hygiene and Therapy.
As part of this year’s National Smile Month, Philips Oral Healthcare are launching #habits4life - to show how good oral health habits lead to a healthier life. Philips want to raise awareness and drive education around 3 key oral care habits. They are; visiting the dentist reguarly, brushing twice-daily with an electric toothbrush, and having a healthy diet. Visit www.habits4life.org.uk to find out more.
By adopting these easy habits you can take control of your health and live healthier and happier lives.
References

