Coronary artery disease
Transforming complex PCI procedures into confident cardiac care
Featured products in Coronary artery disease

Azurion 7 M12 - the heart of our solution for cardiac care
Reduce 17% of procedure time1 by experiencing the outstanding performance of the Azurion 7 M12. This industry-leading image-guided solution supports better patient care and operational efficiency with seamless control from a single tableside touch screen for fast, informed decisions in the sterile field.

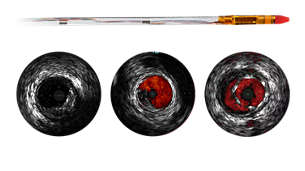
Dynamic Coronary Roadmap - Find your path
Find the right path and improve PCI outcomes. This Philips exclusive solution offers motion-compensated, real-time navigational guidance of coronary arteries and reduces contrast agent by 28.8%²

InstraSight - when every tiny moves matters
The IntraSight applications platform is where imaging, physiology, co-registration3 and software all come together to clearly identify coronary and peripheral artery disease, and allow you to plan the correct course of action for more optimized treatment plans.
Technologies and innovations
-
![Clinically proven Azurion with ClarityIQ technology]()
Providing superb image quality at ultra-low dose settings.
-
Learn how you can guide PCI by using iFR index with coronary physiology for measuring pressure in diagnostic & interventional procedures. See patient outcome data.
Footnotes
[1] Philips whitepaper 12nc 4522 991 30501;Reduction of procedure time by 17% with Philips Azurion in independently verified study; [2] Dynamic Coronary Roadmap versus standard angiography for percutaneous coronary intervention: the randomised, multicentre DCR4 Contrast trial www.documents.philips.com/assets/20240219/0e2a77c4a6904c6eaae3b11b00df6861.pdf [3] Co-registration tools available within InstraSight 7 configuration via SyncVision.
www.philips.com/c-dam/b2bhc/master/landing-pages/azurion/philips-nieuwegein-case-study.pdf
Results are specific to the institution where they were obtained and may not reflect the results achievable at other institutions.